The Periodic Table of Elements
Nov 18 – After the quiz
- Pick up a Periodic Table from the front of the room and write your name in the upper righthand side.
- Use a black sharpie to outline the squares of the most common elements in the galaxy as shown below:
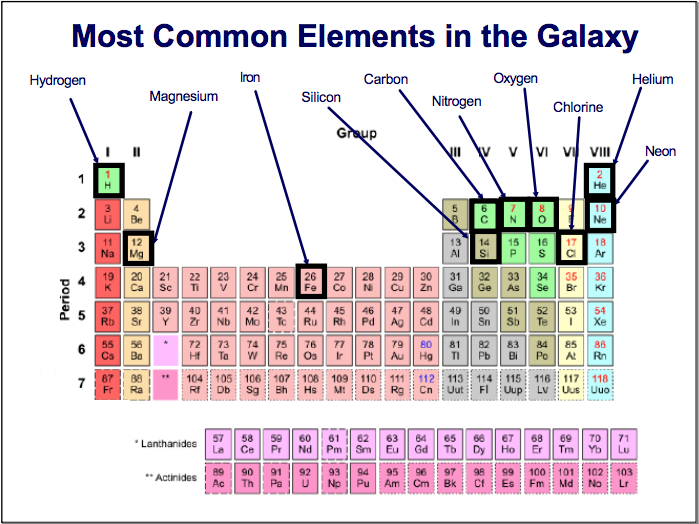
- Use the Periodic Table to complete both sides of the green sheet. Side 1 is a list of the elements who’s symbols you need to memorize. Side 2 is a chart to fill in how many protons, neutrons and electrons each of the elements have in their atoms. If you need a reminder of how to find these, look in your notes from Monday or the presentation listed below by ‘Nov 16 2015.’
Nov 17 2015 – Build an Atom Webquest Reading
Link: https://phet.colorado.edu/en/simulation/build-an-atom
Build An Atom Webquest Answer Key
Nov 16 2015 – History and Organization of the Periodic Table
Keynote: Periodic Table 1 Monday Nov 16
Classwork
17. What did Mendeleev use to organize his first Periodic Table of Elements?
18. What is the symbol for Magnesium?
19. Which element is represented by the symbol C?
20. What period is Hydrogen (H) located in?
21. What group is Sulfur (S) located in?
22. Pick another atom with similar characteristics for each of these elements:
a. Fluorine (F)
b. Oxygen (O)
Homework:
Elements of the Periodic Table Homework
23.How is the modern Periodic Table of Elements arranged?
24.Use your Periodic Table to answer the following questions about Helium (He):
a. Atomic number?
b. Elementsymbol?
c. Atomic mass?
d. Number of protons?
e. Period?
f. Group?
g. Number of electrons in outer shell?
25.Suppose an element had an atomic number of 52. How many protons would that element have?
26.Using your Periodic Table, what element has an atomic number of 65?
27.Using your Periodic Table, which element has more protons: Nickel or Iodine? How do you know?
28.Which element is represented by the symbol W?
29.What period is Silver (Ag) located in?
30.What group is Xenon (Xe) located in?
31.Pick another atom with similar characteristics for each of these elements:
a. Potassium(K)
b. Krypton(Kr)
c. Strontium (Sr)
d. Gold(Au)
Answers
17. He used common characteristics of the elements to sort them into groups. He then noticed that there was a pattern in their atomic weights.
18. Mg
19. Carbon
20. Period 1
21. Group 16
22. For a and b, elements with similar characteristics will be those that are located in the same group:
Fluorine – Chlorine, Bromine, Iodine and Astatine
Oxygen – Sulfur, Selenium, Tellurium and Polonium
23. The modern Periodic Table of Elements is arranged according to Atomic Number (the number of protons in one atom)
24. Atomic # = 2
Element Symbol = He
Atomic Mass = 4
Number of Protons = 2
Period = 1
Group = 18
30. Number of Electrons in Outer Shell = 2 (There are 2 Protons, therefore there are only 2 Electrons. Every other element in Group 18 has 8 Outer Electrons)
25. 52 Protons
26. Terbium
27. Iodine; Iodine has an atomic number of 53 and Nickel has an atomic number of 28. This means that Iodine has 53 protons and Nickel has 28.
28. Tungsten
29. Period 5
30. Group 18
31.
Potassium – Hydrogen, Lithium, Sodium, Rubidium, Caesium and Francium
Krypton – Helium, Neon, Argon, Xenon and Radon
Strontium – Beryllium, Magnesium, Calcium, Barium and Radium
Gold – Copper and Silver



